Did you know that the world’s top drug Humira manufacturer AbbVie has been responsible by the Food and Drug Administration (FDA) for improperly handling death complaints? Also, 482,780 people reported having side effects of Humira injections by January 2020 according to FDA reports?
Adalimumab (Humira) is a part of a class of human monoclonal TNF-α antibody that blocks the effects of TNF-α. These treatments works by suppressing the immune system. While these treatments may be effective in treating symptoms of various diseases (mainly Rheumatoid Arthritis), even though it carries a black-box warning, they put users at a greater risk of severe and even deadly infections and cancers that brings to a list of side effects and practical reviews of Humira.
Why do Humira side effects occur?
Even though there are people who celebrate ten year anniversaries of starting Humira with few minor bumps along the way, you also need to know why people get most of these side effects of Humira due to some of these following reasons.
- Injecting overdose – not using as your doctor advises you to use
- Have an infection or being treated for an infection before treatment
- Use Humira at the same time with other biologics
- Have or have had cancer or a heart failure
- Have diabetes
- You are pregnant or breastfeeding during this treatment period
- Live or have lived in a certain area where there an increased risk of getting fungal infections such as histoplasmosis, coccidioidomycosis, or blastomycosis
- Have numbness or a nervous system disease such as Guillain-Barré syndrome or multiple sclerosis
For your information: Make sure to tell your doctor about all the medicine you take and all the health conditions you have or had before treatment starts.
IMPORTANT: According to humira.com you should not take HUMIRA with ORENCIA® (abatacept), KINERET® (anakinra), REMICADE® (infliximab), CIMZIA® (certolizumab pegol), SIMPONI® (golimumab) or ENBREL® (etanercept). Tell the doctor if you have ever used IMURAN® (azathioprine), RITUXAN® (rituximab), or PURINETHOL® (mercaptopurine, 6-MP) before.
Severe side effects of Humira
Nowadays, many attorney groups are waiting for you to reach out to them to file a lawsuit against Humira because of these serious injuries you or your loved one experienced.
The USA Food and Drug Administration (FDA) has recognized Humira as a dangerous drug that can cause Humira side effects like depression, anxiety, and hair loss, including risks for potentially life-threatening side effects.
No surprise that it also at the top of the list of drugs with the most adverse event reports reaching the FDA.
Facts: In September 2018, the State of California filed a lawsuit against AbbVie, alleging the manufacturer gave “illegal kickbacks” to most physicians to encourage them to prescribe Humira to patients.
In addition to tuberculosis (TB) black-box warning, today, Humira carries a warning for invasive fungal infections, such as histoplasmosis caused by Histoplasma, as well as Legionella and Listeria infections. But this was not always the case. For years, Humira pen’s black-box warning only included the risk of TB.
If you have any of these below severe side effects and symptoms of Humira, stop taking it and call your doctor immediately!
- Tuberculosis (TB)
- Cancer
- Invasive fungal infections
- Severe allergic reactions
- Legionella and listeria bacteria
- Hepatitis B reactivation
- Heart failure
1. Tuberculosis (TB)
Tuberculosis (TB) is a potentially severe infectious condition that primarily affects your lungs. The bacteria that cause this condition can also spread from one person to another through tiny droplets released into the air from sneezes and coughs.
Facts: This infection is rare in developed countries that began to increase in 1985 due to the emergence of the virus HIV that weakens a person’s immune system so it can’t react to TB germs.
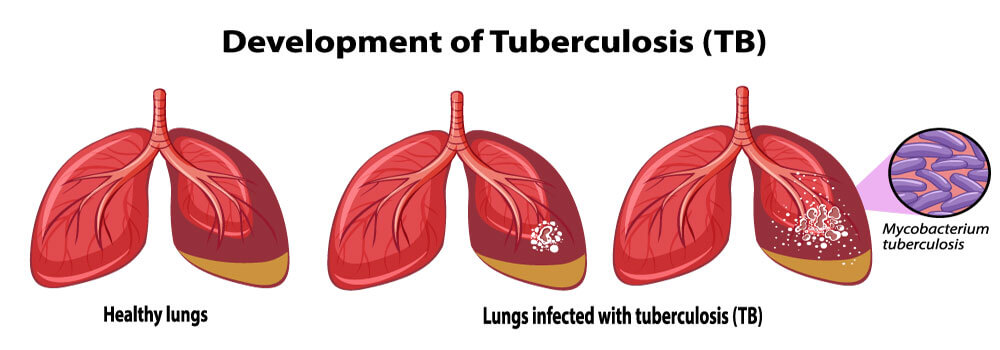
Many patients have reported about the increased risk of tuberculosis (TB) who treated with TNF-α antagonists, the issue that has been highlighted in the Humira’s black box warning. This review aimed to assess the risk of TB in patients undergoing Humira treatment.
A real-world story:
This story is about a 59-year-old woman, Caucasian, with a previous history of osteoarticular TB at the age of 7 years old.
She was diagnosed as Behcet’s disease with a 23-year-old history of outbreaks of oral ulcers. Since the age of 55 years, she started with severe gastrointestinal manifestations, irresponsive to corticosteroids and infliximab. She began adalimumab treatment three years ago with clinical improvement. Before Adalimumab therapy, she completed nine months of isoniazid for latent tuberculosis.
The patient suffered from high fever, asthenia, and night sweats for one month, without other symptoms associated. Blood tests were normal, with a slight protein C elevation. Blood and urine cultures were sterile. Pulmonary X-ray and abdominal ultrasound didn’t reveal any issues.
After two weeks of the first symptoms, she complained about left pain without trauma associated. Computed tomography (CT) scan showed a left psoas muscle mass (diameter 4 – 14 cm with calcifications).
She was submitted to percutaneous drainage of the abscess, and the microbiologic study revealed nucleic acid amplification (NAA) test positive for Mycobacterium tuberculosis, which also detected in cultures.
The patient began treatment with the classic association of antibiotics that included isoniazid, rifampicin, pyrazinamide, and ethambutol for nine months with complete resolution of the abscess.
Symptoms of active TB include:
- Coughing that lasts three or more weeks
- Coughing up blood
- Night sweats
- Pain with breathing or coughing
- weight loss
- Fatigue
- Fever
- Chills
As mentioned in the Humira’s black-box patients are advised to be tested for TB before starting any Humira shot. Patients who positive for TB should start treatments for the disease before start treatments with Humira.
Doctors should pay more attention to all the patients for signs and symptoms of TB during the Humira treatments; even the initial latent TB test is negative.
2. Cancer
Humira has been linked to serious side effects, including increased risk of a rare but usually fatal form of cancer.
In 2008, the FDA investigated Humira for 30 reports of childhood cancer and its links to lymphoma, leukemia, and melanoma in children, as we discuss further below. Yes, it’s lymphoma.
That discovery resulted in an update of the black-box warning for TNF-blocker medications.
Here is a discussion about the increase in Cancer risk from John Kisiel, M.D., a gastroenterologist at Mayo Clinic, as in the video below!
Lymphoma, leukemia, and melanoma
Reports of cancer were reported to the FDA’s adverse event reporting system from the beginning of 1998 after the first TNF blocker hit the market.
About half of the cancer reports were about lymphoma. Including both Hodgkin’s and non-Hodgkin’s lymphoma, and other cancers reported included leukemia, melanoma, and solid organ cancers.
After many years of investigation in the follow-up announcement in 2009, the FDA said that they analyzed and concluded that there is an increased risk of Lymphoma, leukemia, and melanoma with the use of TNF blockers, including Humira.
Hepatosplenic T-cell lymphoma (HSTCL)
Hepatosplenic T-cell Lymphoma (HSTCL) is a rare type of T-cell lymphoma (a type of white blood cell or lymphocyte) generally found in 20-29 old males that have been taking the treatment for 6 to 12 months, also take medication Remicade (Infliximab) and have Ulcerative colitis (colitis ulcerosa).
It is aggressive and even fast-growing cancer that affects the liver, spleen, and sometimes the bone marrow. In most cases, HSTCL is usually fatal. Symptoms include fever, fatigue, jaundice, and infections. Tests often reveal abnormal liver function and reduced peripheral blood cells.
Symptoms of active HSTCL include:
- Ulcerative Colitis (inflammatory bowel disease (IBD). it causes swelling, ulcerations, and loss of function of the large intestine)
- Colitis (inflammation of the colon)
- Inflammatory Bowel Disease
- Irritable Bowel Syndrome
- Chemotherapy
Unfortunately, because of the aggressive nature of HSTCL, most cases are fatal. However, there are treatment options, but aggressive cancer requires aggressive treatment. Even though patients need to back on their feet, unfortunately, sometimes even the most aggressive treatment options can not cure HSTCL when it comes to the side effects of Humira.
3. Invasive fungal infections
A boxed warning regarding the risk for invasive fungal infections must be added to the prescribing information for TNF–α blockers.
Sept. 4, 2008 – The FDA ordered stronger warnings about the risk of fungal infections, especially one called histoplasmosis, in people who take Humira treatments.
The FDA has received 240 reports from patients who are taking TNF blockers who developed histoplasmosis during the treatment period. This condition is a fungal infection that starts as a respiratory infection and has chances to spread throughout the body.
Jeffrey Siegel, MD, clinical team leader, mentioned that 45 patients out of 240 histoplasmosis patients have died, including at least 12 people who hadn’t been diagnosed with histoplasmosis right away.
Users of Humira have been asked to call their doctor if they develop persistent cough, fever, fatigue, or shortness of breath during the treatment periods. And also, doctors have been advised to monitor their patients on TNF blocker drugs for possible fungal infections and to start antifungal therapy if they are needed.
4. Severe allergic reactions
People have experienced anaphylaxis ( life-threatening allergic reaction) and angioneurotic edema (swelling) following Humira administration.
Symptoms of a serious allergic reaction include trouble breathing, hives, numbness, and swelling of the face, eyes, lips, or mouth. If patients experience signs of an allergic reaction, they should call their doctor or get medical help immediately.
5. Legionella and listeria bacteria
Sep. 7, 2011 – FDA issued a drug safety communication alerting the healthcare professional that agency was updating the black-box warning regarding TNF-–α blockers due to the increase of the risk of infections legionella and listeria bacteria.
Listeria infection, which is also called listeriosis, constitutes a risk for children, elders, and those with weakened immune systems. Even though there are no records about infection reports according to FDA about Listeria, but they mentioned that a search of English-language medical literature turned up 26 reports about listeria infection, which led to seven deaths.
Blood tests are required to diagnose listeriosis, which can treat with antibiotics.
Legionella disease is a serious life-threatening infection, and according to U.S. Centers for Disease Control and Prevention (CDC), 8000 to 18000 people hospitalized each year due to this disease.
Legionella bacteria cannot be transmitted from one person to another but can be found in warm water and can be present in hot tubs, hot water tanks, cooling towers, and large plumbing or air conditioning systems.
6. Hepatitis B reactivation
Hepatitis B is a form of a viral infection that attacks the liver with the hepatitis B virus (HBV), an infectious disease that kills more than 780,000 people worldwide each year. It can lead to chronic infection to put people at high risk of death from cirrhosis and liver cancer.
People who carry the hepatitis B virus (HBV) in their blood should be extra cautious when they decide whether to use Humira because the virus can become reactive during treatment.
According to Humira, in some instances, HBV reactivation occurring in conjunction with TNF blocker therapy has been fatal. Therefore patients should take blood tests before, during, and a few months after the treatments.
7. Heart failure
Within many side effects of Humira reported by the FDA, heart failure is also on the list. The manufacturer AbbVie includes heart failure as a side effect, even though it would appear to be a rare condition based on a literature review that acknowledges the risks of heart failure associated with Humira in a press release.
In 2013, Yale researchers Ritesh Kohli and Karim Namek authored a report on Humira, and in the “Background” section, they mentioned that the known secondary effects related to adalimumab included congestive heart failure.
Side effects of Humira – Conclusion
Humira appeared to be effective in many patients, even though not everyone who gets treatments with the drug sees a dramatic improvement. Various studies and practical reviews have been put together to examine the effectiveness of Humira when it comes to treating various conditions, mainly Rheumatoid Arthritis.
There are obvious common side effects of Humira as well as severe life-threatening conditions as above.
You must be aware of the warnings and symptoms of Humira side effects. So, you can tell your doctor if something is wrong or if you are having any health issues you think could be related to treatments.
Patients must first learn how to administer Humira correctly and safely by a qualified person. Ask your doctor if you’re unsure of anything, or you haven’t been taught how to do a subcutaneous injection properly.

